We are pleased to inform you that the Paris Commercial Court approved the sale of the Pixium Vision business to Science Corporation.
For all questions, please write to pixium@science.xyz
ABOUT PIXIUM VISION
PIXIUM VISION harnesses rapid advances in visual processing, micro-electronics, opto-electronics, neurobiology, and intelligent software algorithms to develop Bionic Vision Systems utilizing its competencies in machine brain interface and artificial intelligence.
These Bionic Vision Systems are aimed at compensating for profound vision loss and improving the independence, mobility and quality of life for patients suffering with retinal degenerative diseases.
FACE THE FACTS
For society, blindness is a major economic and social issue leading an increase in direct healthcare costs and indirect costs (lost productivity, costs of care by non-professionals). Treatment for blindness represents a major unmet medical need throughout the world.
Treatment for blindness represents a major unmet medical need throughout the world.
There is currently no cure for blind patients and the consequences of blindness to sufferers include:
Risk of Depression
There is a high risk of depression
Tens of Billions
Blindness costs tens of billions of USD in Europe and the USA3
Quality of Life
Deterioration of quality of life with earlier admission to nursing homes and long-term care facilities
Premature Death
There is a two fold increase in the risk of premature death
Sources
1 WHO 2014 Eye Care fact sheet 282 http://www.who.int/blindness
2 Wong, WL et al., Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health
3 NORC University of Chicago – The Economic burden of vision loss and eye disorders 2013 http://www.norc.org/Research/Projects/
INTERNATIONAL TEAM
Pixium Vision is supported by a prestigious scientific advisory board, and partners which are international, multidisciplinary, spanning basic science to medical expertise, and highly recognized and respected in their fields.
RECENT CLINICAL TRIALS

PIXIUM VISION Continues to Evolve
PRIMA-US-FS clinical study in U.S. and PRIMA-FS clinical study in France are two similar feasibility clinical studies to evaluate the Prima System.
PRIMA-FS clinical study in France successfully completed implantation in 5 patients.
– The first results at 6 months from the study in France were reported during the American Academy of Ophthalmology AAO annual meeting held in Chicago in July 2019
– One year follow-up results were announced by the company in July 2019 and have been reported at the Eye and the Chip meeting in Detroit in November 2019
The results observed in the feasibility study make it possible to consider starting a pivotal study which aims to demonstrate the system’s performance.
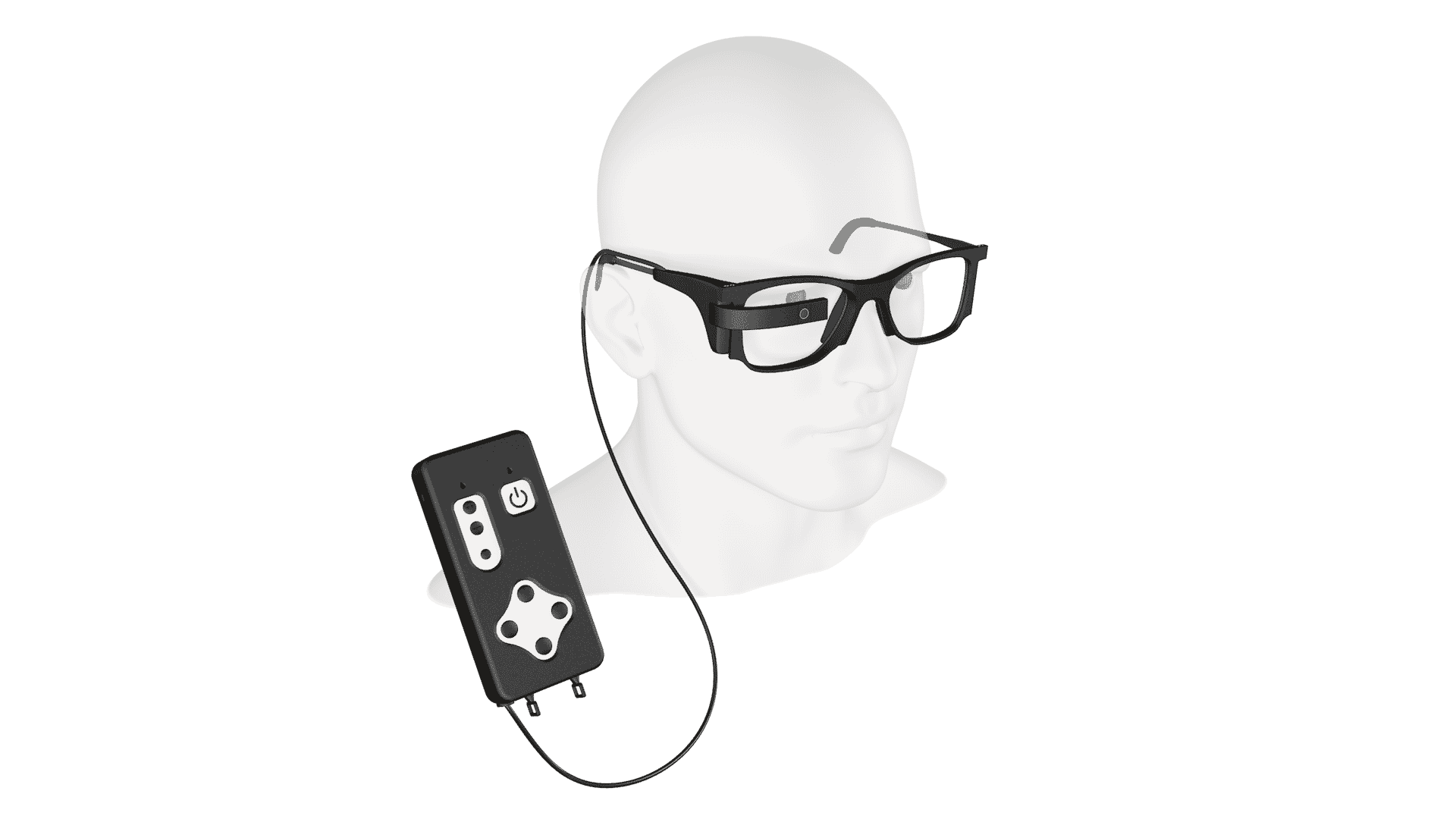
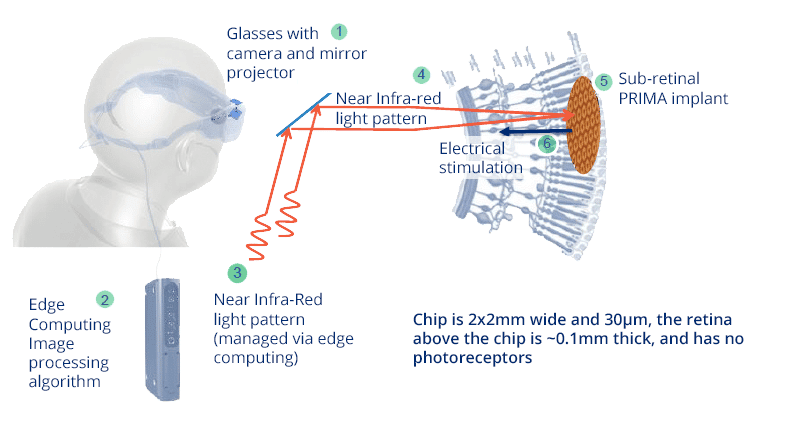
THE PRIMA TECHNOLOGY
RECENT NEWS & PUBLICATIONS
STOCK DATA
Ticker: ALPIX

Pixium Vision shares are eligible for the French tax incentivized PEA-PME and FCPI investment vehicles