PRIMAvera PIVOTAL STUDY ON DRY AMD (EUROPEAN Study)
INDICATION: Advanced Atrophic Dry Age-related Macular Degeneration (dry-AMD)
DEVICE: The Prima System is a Bionic Vision System, including a totally wireless implantable photovoltaic sub-retinal implant, is designed to elicit useful functional prosthetic vision.
PRIMA aims to partially restore central vision that was lost due to atrophic age-related macular degeneration
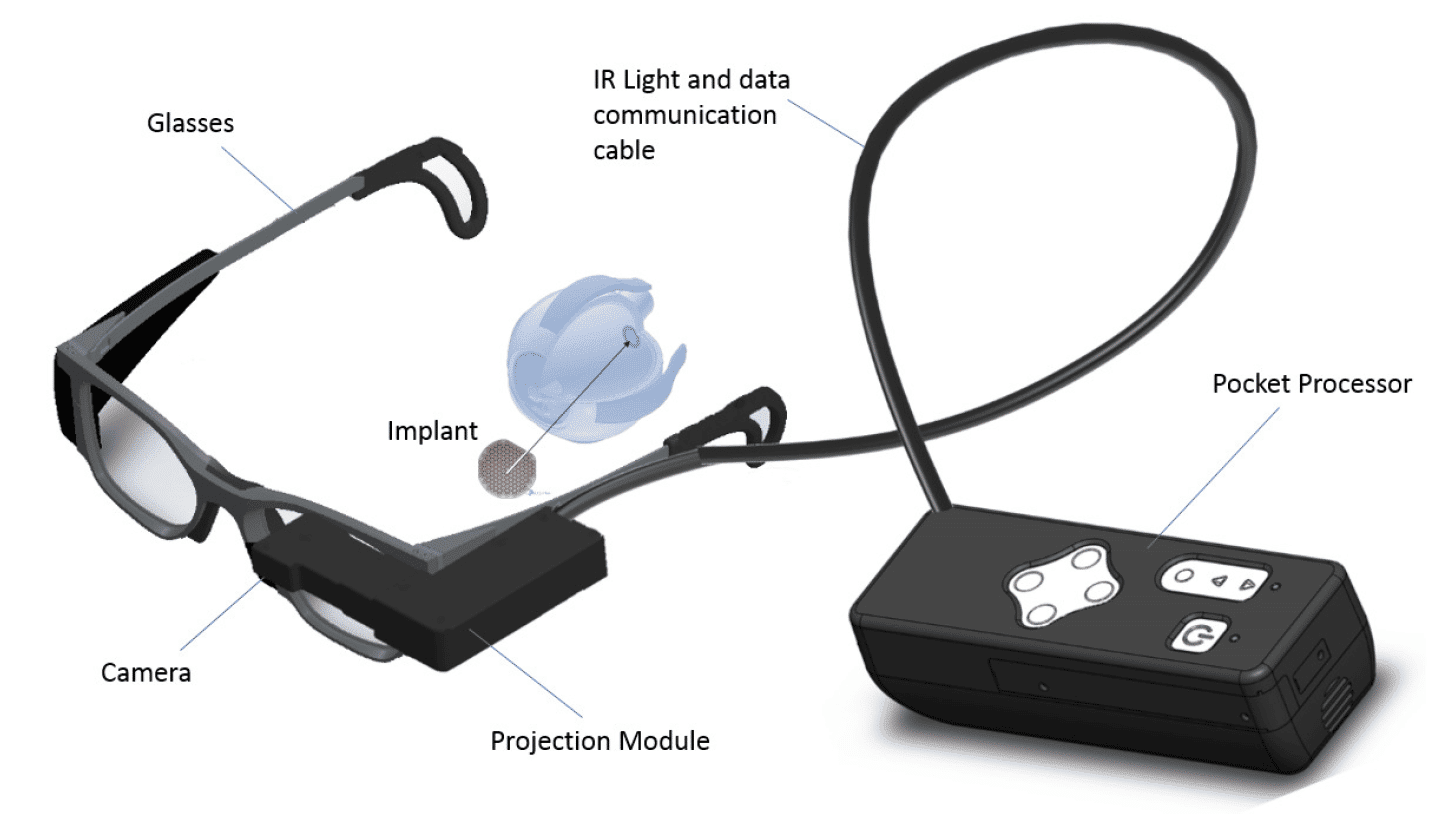
The PRIMA system consists of three main elements:
- The tiny wireless retinal implant
- A pair of glasses with an integrated camera and a projection module
- A pocket processor connected to the projection module
Clinical Study
PRIMA has been tested in the laboratory and in two ongoing feasibility studies. Preliminary results indicate that the device has the potential to restore vision in a way that letters and words could be recognized again.
The PRIMA system is now under clinical testing within the so called “PRIMAvera” study. The aim of this clinical study is to demonstrate the performance and to confirm the safety of the device.
Primary endpoint: Assessment of visual acuity measured at 12 months
Information on the study:
More information on ClinicalTrials.gov
ClinicalTrials.gov Identifier: NCT04676854
Enrolment for the PRIMAvera clinical trial has been completed and the implanted patients are currently in the clinical follow-up period.
OVERALL NUMBER OF PATIENTS: 38
Criteria to participate in the study
- Atrophic AMD (also known as geographic atrophy)
- 60 years or older
- Visual Acuity of logMAR 1.2 (20/320) or worse in the worse seeing eye.
For other criteria, please visit www.clinicaltrials.gov
Participating clinics in Europe
Germany
Universitätsklinikum Aachen
Klinik für Augenheilkunde Aachen, 52074 Germany
Contact : Prof. Peter Walter
Universitäts-Augenklinik Bonn
Bonn, Germany, 53127
Contact: Prof. Frank Holz
Universitätsklinikum Hamburg-Eppendorf (UKE)
Augenklinik Hamburg, Germany, 2025
Contact: Prof. Martin Spitzer
Klinikum Ludwigshafen-Augenklinik
Ludwigshafen am Rhein, Germany, 67063
Contact: Prof. Lars-Olof Hattenbach
Augenklinik der Ludwig-Maximilian Universität München
Munich, Germany, 80336
Contact: Prof. Siegfried Priglinger
KNAPPSCHAFTSKLINIKUM SAAR GMBH Augenklinik Sulzbach
Sulzbach/Saar, Germany, 66280
Contact: Prof. Boris Stanzel
Universitätsklinikum Tübingen
Tübingen, Germany, 72076
Contact: Prof. Karl Ulrich Bartz-Schmidt
Universitätsklinikum Schleswig-Holstein
Klinik für Augenheilkunde
Ratzeburger Allee 160
23538 Lübeck
Contact: Prof. Dr. med. Salvatore Grisanti
Universitäts-Augenklinik Münster
Albert-Schweitzer-Campus 1
Gebäude D15
48149 Münster
Contact: Pr. Eter Nicole
Universitätsklinikum Ulm
Prittwitzstraße 43
89075 Ulm
Italy
Università di Roma Tor Vergata – Policlinico Tor Vergata (PTV), Dipartimento di Medicina Sperimentale – Oftalmologia
Roma, Italy, 00133
Contact: Dr. Andrea Cusumano
Netherlands

Rotterdam Eye Hospital
Rotterdam, Schiedamse Vest 160, Netherlands, 3011 BH
Contact: Dr. Koen van Overdam
Spain
Instituto de Microcirugía Ocular de Barcelona
Barcelona, 08035 Spain
Contact: Dr. Rafael Navarro
United Kingdom
Moorfields Eye Hospital NHS Foundation Trust
162 City Road, London EC1V 2PD, UK
Contact: Mahi Muqit
France
Ongoing feasibility studies suggest that the PRIMA system can potentially improve visual function and functional vision in visual impaired patients